Choose the correct statement(s) among the following: FeCl4- has tetrahedral geometry. Co(en)NH32Cl2+ has 2...
Choose the correct statement(s) among the following:
Solution:
Oxidation number of Fe atom
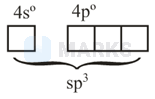
is having tetrahedral geometry
have three geometrical isomers.

is hybridized with 5 -unpaired electrons. While is hybridised with zero unpaired electrons.
has hybridization.
















 JEE Main
JEE Main JEE Advanced
JEE Advanced NEET UG
NEET UG BITSAT
BITSAT COMEDK
COMEDK VITEEE
VITEEE WBJEE
WBJEE
Join the conversation